Biokin Pharmaceutical’s iza-bren (BL-B01D1), a proprietary, first-in-class and new-concept EGFR x HER3 bispecific ADC, has met its primary endpoint in the planned interim analysis of its Phase III trial (Study Protocol No.: BL-B01D1-303). The trial is for patients with recurrent or metastatic nasopharyngeal carcinoma who have progressed after treatment with both a PD-1/PD-L1 inhibitor and at least two lines of chemotherapy, including a platinum-based regimen.
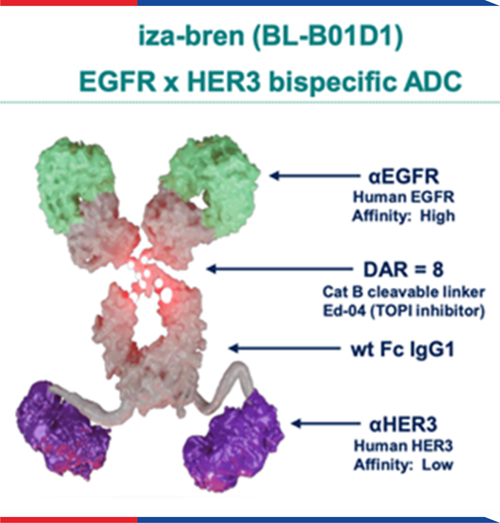
As the world’s first-in-class EGFR x HER3 bispecific ADC, iza-bren (BL-B01D1) has demonstrated promising preliminary efficacy in patients with locally advanced or metastatic solid tumors, highlighting its potential for clinical application.
Currently, 10 Phase III clinical studies are underway for iza-bren (BL-B01D1) across multiple solid tumours, including NSCLC, SCLC, nasopharyngeal carcinoma, breast cancer, urothelial carcinoma, and esophageal cancer. Among them, 5 of these indications have been granted Breakthrough Therapy Designation by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA).
Furthermore, iza-bren (BL-B01D1) is also undergoing over 40 clinical trials in China and the United States across various tumor types. As multiple pivotal Phase III studies continue to progress and more results are released, the efficacy benefits of iza-bren (BL-B01D1) will be further solidified.


