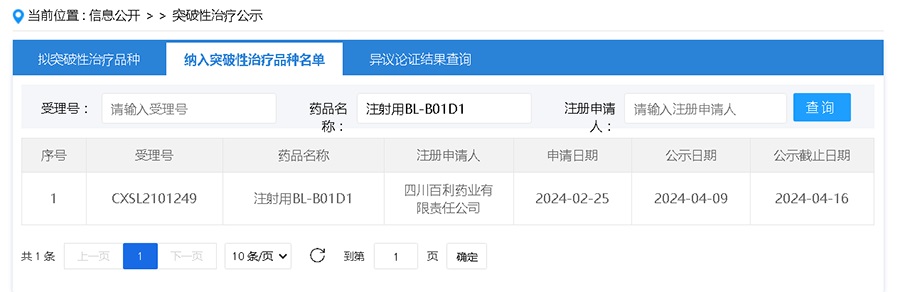
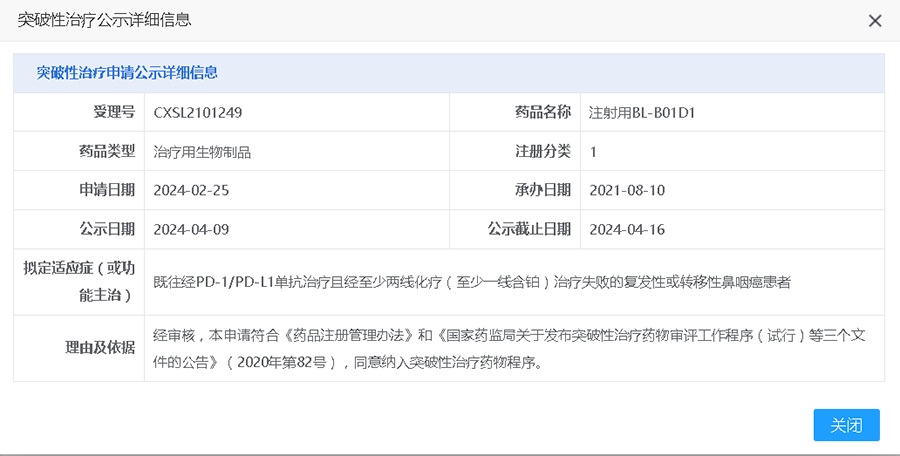
The innovative biological drug BL-B01D1 (EGFR×HER3-ADC) for injection independently developed by Biokin Pharmaceutical for the treatment of patients with locally advanced or metastatic nasopharyngeal carcinoma receiving end-line therapy has been included in the list of breakthrough treatment drugs by CENTER FOR DRUG EVALUATION, NMPA (hereinafter referred to as "CENTER FOR DRUG EVALUATION"), and has recently been publicized.